Quite Interesting Calculations: The Iron Catastrophe
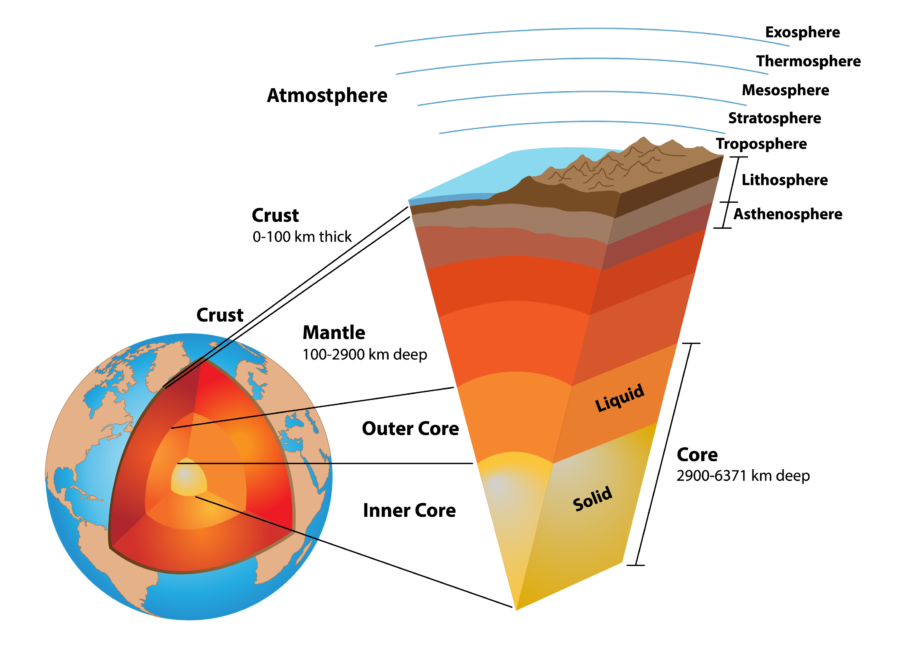
Figure 1: Layers of the earth with approximate depths.
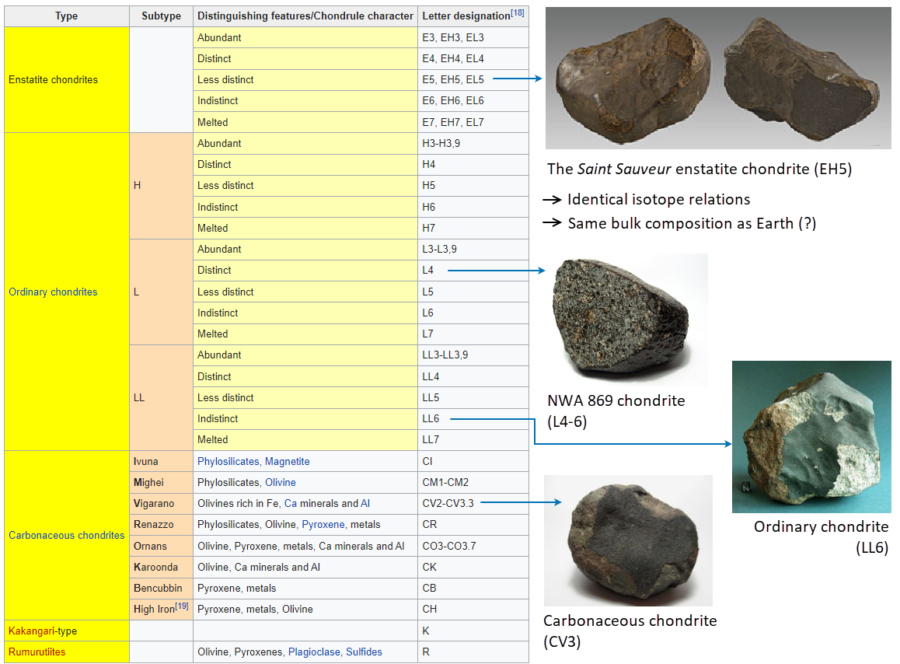
Figure 2. Chondrites, including the enstatite chondrites, which are assumed to have a similar composition as the bulk earth. Table and all pictures taken from Wikipedia: https://en.wikipedia.org/wiki/Chondrite.
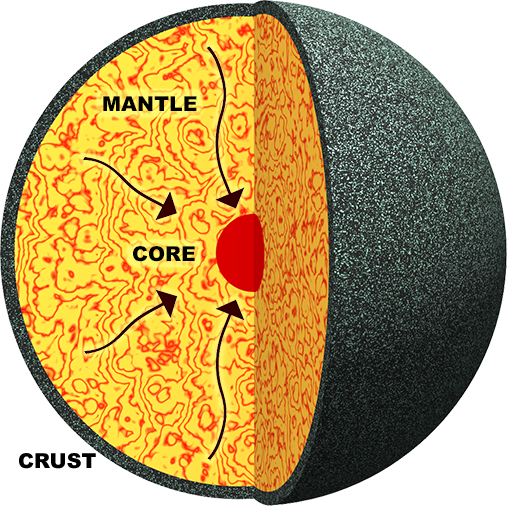
Figure 3. Illustration of the formation of two immiscible liquids during the early formation of the Earth. The dense metallic liquid sinks to the centre, thereby forming the liquid metallic core of the Earth.
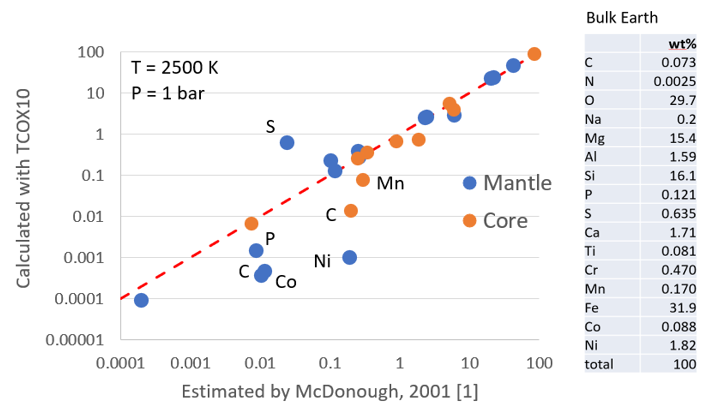
Figure 4. Comparison of the core and mantle composition as calculated with Thermo-Calc and TCOX10 assuming T = 2500 K and P = 1 bar with the compositions estimated by McDonough [1]. The “Bulk Earth” composition is also from [1].
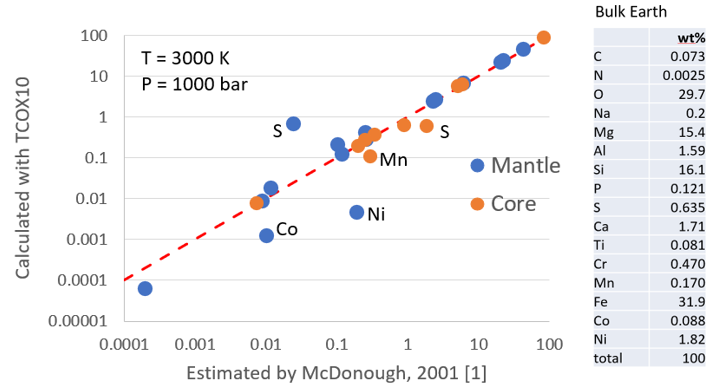
Figure 5. Comparison of the core and mantle composition as calculated with Thermo-Calc and TCOX10 assuming T = 3000 K and P = 1000 bar with the compositions estimated by McDonough [1]. The “Bulk Earth” composition is also from [1].
