About the Equilibrium Calculator
Four Calculation Types
Single Equilibrium
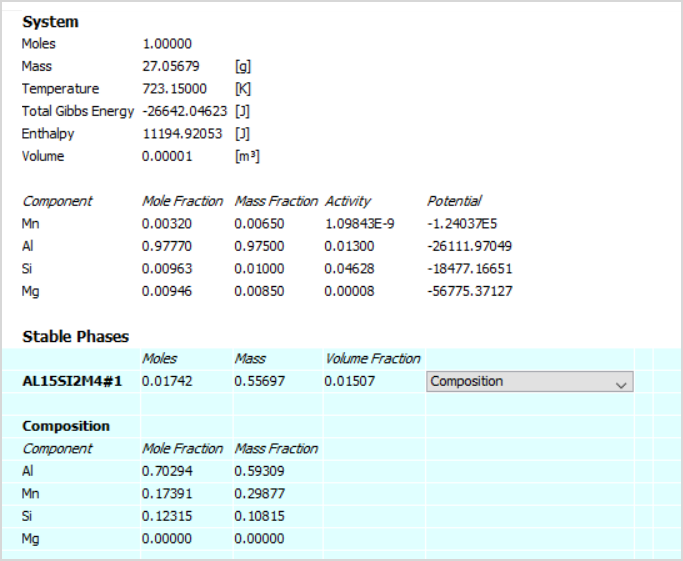
This calculation type allows you to calculate the equilibrium for a specific set of conditions. The results of the calculation are provided in the form of a table, which shows the stable phases that are predicted to form under those conditions and the composition of those phases.
The table shows partial results of an equilibrium calculated at 450°C for Al-1Si-0.85Mg-0.65Mn (AA6082). Stable phases not shown in the image are FCC_A1 and MG2SI_C1.
One Axis
This calculation type allows users to vary (or step in) one of the conditions specifying equilibrium. In this way, a series of equilibrium calculations is made where one of the conditions is changing, in steps, from one value to another. The results can be provided in a table or as a plot, as is the case for the example, which shows the volume-fraction of all stable phases present in a commercial Al-alloy, as a function of temperature.
A range of other properties can be plotted in a similar way, for instance composition of phases, heat capacity, density, and many more.
The plot shows a one-axis calculation of volume-fraction of stable phases as a function of temperature for Al-5.6Zn-2.5Mg-1.6Cu-0.2Si-0.15Mn (AA7075).

Grid
When selecting this calculation type, two conditions specifying the equilibrium are varied with equidistant steps. The result is provided as a plot, such as in the example here showing the viscosity in an Al-SI-Mg-Mn melt, just above the liquidus temperature.
The plot shows a grid calculation of dynamic viscosity calculated at 680°C for Al-1Si-yMg-xMn alloys.

Phase Diagram
In this calculation type, two conditions are specified and varied, just as with a grid calculation, but in this case phase boundaries (or zero phase fraction lines) are tracked and followed to generate a phase diagram. The result is provided as a plot, as shown in the example, where the Si content is varied on the x-axis and temperature on the y-axis.
The plot shows a phase diagram, also known as an isoplethal section, for Al-xSi-0.85Mg-0.65Mn.

Phase Diagram in Isothermal Conditions
Users can generate another type of phase diagram by varying the amounts of two elements at a constant temperature (isothermal conditions). Such an example is shown here, which is a phase diagram calculated at 1100°C for a ternary Fe-Cr-Mo system.
The plot shows a ternary phase diagram, also known as an isothermal section, for Fe-Cr-Mo at 1100°C.

Learn More about the Equilibrium Calculator
Calculating a Single-point Equilibrium
Stepping in Temperature in the Fe-C System