Sustainability in Materials

Influence of oxygen on the phase boundaries in the Ti-Al system, calculated using the TCTI Database, which was updated to use the modified modeling from the ADVANCE project.


Left: Equilibrium simulation showing Ni/Fe recovery (wt%) from the melt at 1600 ⁰C. Right: Chemical evolution of silicate melt upon exposure to hydrogen. The figure is based on Manzoor et al. [2] but was but was recalculated using only the TCOX Database.

Left: Equilibrium phase diagram for AlZnMg alloy with and without impurities (Fe, Si). Addition of small amounts of impurities (0.5 wt% each) stabilizes Al13Fe4 and Mg2Si phases, which cannot be treated with standard solution heat treatments. Right: Scheil simulations (non-equilibrium) show the solidification route upon casting.

Left: Ca and Na content in the Aluminum melt when reacting with a KCl-MgCl2 flux with varying MgCl2 content. As seen, Ca and Na can be removed with increased MgCl2. Right: Mg content in the Aluminum melt when reacting with a KCl-MgCl2 flux with varying MgCl2 content. As seen the Mg content is maintained at ~4.5wt%.

Example on A3003 alloy (with 0 Mn and 1.5 wt% Mn) showing the equilibrium amount of beta-Al9Fe2Si2 with increasing Fe amount and temperature.


Solidification diagram for Fe-5Cu and Fe-5Cu-0.7Sn alloys (left) and Experimental and simulated segregation for Cu and Sn for these alloys (right). Mehta, B., Yang, X., Höglund, L., Mu, W., & Hedström, P. (2025). Toward Scrap-Tolerant Steels: Investigating the Role of Cu and Sn Micro-Segregations on Solidification Microstructure and Cracking [Figure 9]. Journal of Sustainable Metallurgy, 11, 1908-1921. https://doi.org/10.1007/s40831-025-01093-4. Licensed under CC BY 4.0.

Simulation of Hematite (Fe-O alloy) with Cu addition in a Ar-10wt%H2 reducing environment showing ~70% Cu loss to Gas phase at 1850 ⁰C when H2 is introduced. The figure is based on Filho et al [7] but was recalculated using only the TCOX Database.
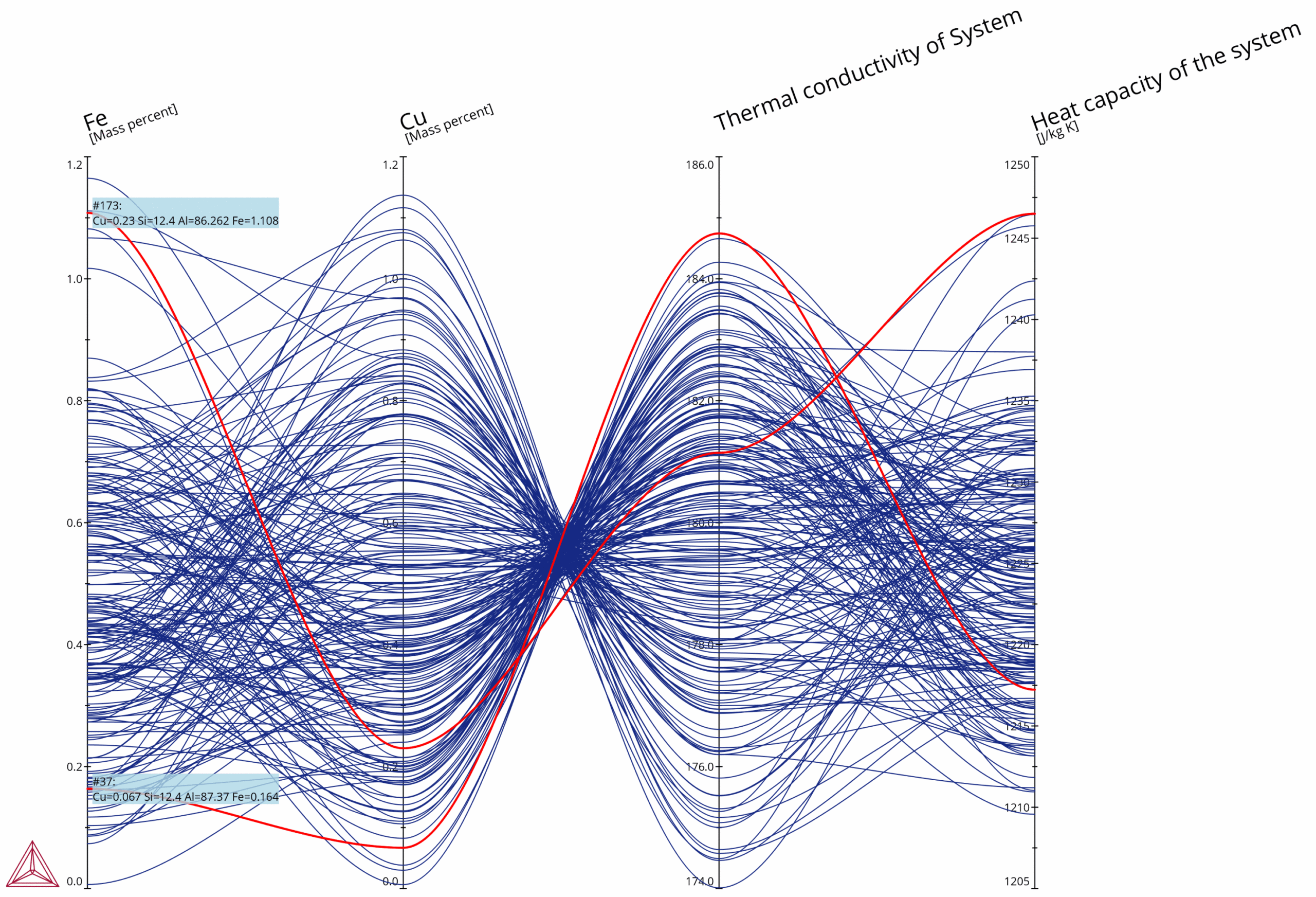
Parallel coordinates plot showing the composition dependence on properties relevant to thermal storage, namely Thermal conductivity and Heat capacity

WISE Funding to Drive Sustainable Li-ion Battery Innovation

Use Cases
Webinars on Sustainability
Learn more about how Thermo-Calc can help you reach your sustainability goals with this collection of webinars, presented by both Thermo-Calc employees and users who discuss how they use Thermo-Calc in their own work.

LET'S WORK TOGETHER TO
Reach Your Sustainability Goals
We are proud to enable sustainable innovation, and we are continuously developing our tools to better support our customers and partners who share our sustainable values and goals. If you want to collaborate with us on projects or use cases, we encourage you to reach out. Or send a consultation request to learn more about how Thermo-Calc products can support you in your work with sustainability.