CALPHAD Assessments
In 1908, seven years after Bakhuis Roozeboom’s treatise “Die Heterogenen Gleichgewichte”, which brought attention to the connection between phase diagrams and thermodynamics, another Dutch scientist, van Laar, showed how the shape of typical T,x phased diagrams can be predicted from expressions representing the Gibbs energy of the individual phases as functions of T and composition, x.
In the following years, there were a few occasions when people used this relation between thermodynamics and phase diagrams to go the other way and derive useful thermodynamic information from experimental information on phase diagrams. Yet it seems to have taken about 30 years until somebody collected information on both thermodynamics and phase diagrams to assess the properties of a system from which thermodynamic quantities as well as phase equilibria could be recalculated with increased confidence.
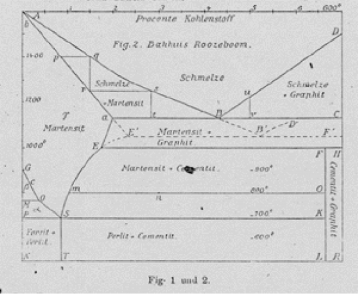
In 1937 Carl Hugo Johansson of the physics department of KTH in Stockholm analysed the properties of part of the Fe-C system and published what may have been the first modern CALPHAD assessment. Instead of asking why it took so long, we may ask why it finally happened. Why did a physicist with sufficient understanding of thermodynamics finally take an interest in such a practical matter as a phase diagram?
The explanation is simply that the Swedish “King”, i.e. the government, had decided that there should be a chair in “Metallografi”, a field that today is called Physical Metallurgy. It was given to the department of Mining and Metallurgy at KTH and Johansson decided to apply. In order to qualify he wanted to show that the new field could profit by academics exploring its scientific basis. He thus assessed the Fe-C system and applied the results to explain the transformation to martensite, a topic of major interest to the Swedish steel industry.
Johansson was not appointed to the chair and saw no reason to continue this new line of research, so it was not taken up again until well after the war. It is intriguing to imagine, however, how much quicker the field of CALPHAD could have developed if Johansson had been appointed.
This article is the second in a three-part series tracing the evolution of CALPHAD from the Gibbs Phase Rule to the first lattice stabilities. Click below to view the other articles in the series: